FISH-SISH-CISH to unravel the morphological data of your samples
Optimized ISH assays
CellCarta has an extensive experience with in situ hybridization (ISH), with assays deployed in exploratory, clinical and companion diagnostic projects. In addition, we have deployed cytogenetic ISH assays for diagnosis, prognosis and therapeutic surveillance of cancer patients.
Our team offers customized assay development with different automated staining platforms. Whether for existing or de novo ISH assays, single target or multiplex combined with IHC detection on the same slide, our services include providing the best quality target probe (in part through our partnership with manufacturers), proper selection of the platform, selection of control tissues and pre-treatment conditions to obtain the best signals.
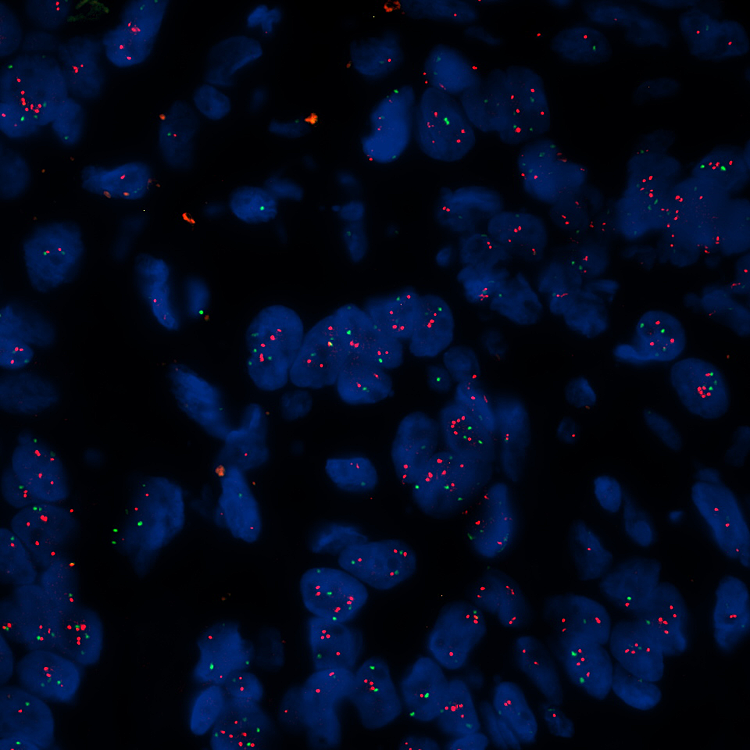
Image: HER2 FISH
Our ISH technology
The ISH technology offers a rich set of morphological detail however, it is typically quite a labor-intensive process. At CellCarta, we have assembled a chain of semi-automated equipment to standardize this process as effectively as possible.
- ISH assays are performed on an automated system with hybridization steps on calibrated heating modules using the ThermoBrite Heating Chamber.
- Fluorescence ISH (FISH) assays are semi-automated using the Abbott VP2000.
- Silver-enhanced ISH (SISH) assays are performed on the automated system Benchmark XT and Ultra (Ventana, Roche) which can handle up to 30 slides in one run.
- Multiplex assays combining IHC and chromogenic ISH (CISH) on the same slide are performed on the Leica Bond autostainer.
Evaluation and quantification of FISH signals is carried out using Zeiss Axioplan microscopes and BioView system (BioView Ltd) with motorized stages ensuring accurate imaging of your specimens. All equipment has been qualified extensively and is submitted to regular quality checks. After each intervention, instruments are re-qualified before release. Chromogenic ISH stained slides are captured using high-resolution whole slide digital imaging.
DNA FISH
CellCarta has been developing numerous FISH assays for exploratory and late phase clinical trials. In addition, we have deployed cytogenetic FISH assays for diagnosis, prognosis and therapeutic surveillance of cancer patients
- FISH assays are performed on an automated system with hybridization steps on calibrated heating modules. This semi-automated process can handle up to 50 slides in one run.
- SISH assays are performed on the automated system Benchmark XT and Ultra (Ventana, Roche) which can handle up to 30 slides in one run.
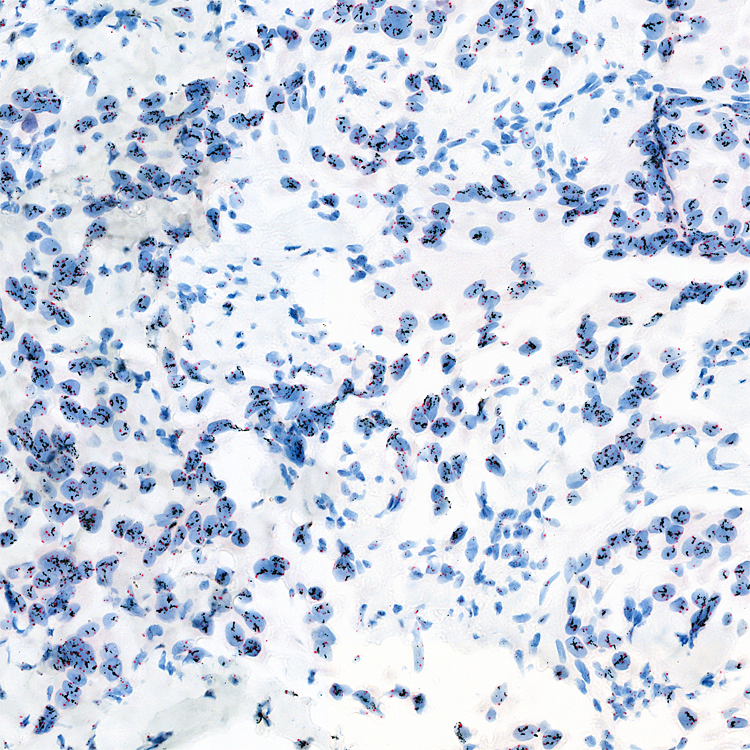
Image: HER2 SISH
mRNA ISH
Cell experiments:
CellCarta has extensively evaluated the new RNAscope® (ACD) nucleic acid in situ hybridization technology. This method is now valid for use on FFPE cell preparations at CellCarta and can be requested for any target of interest to be determined in your cells.
FFPE tumor tissue experiments:
The approach for validation of this methodology on FFPE tumor tissue samples is in line with our standard protocols. However, the technology requires custom validation to fine tune the procedure to the needs of your specific specimens. When you approach us for your mRNA ISH assay, your specimens will be tested during the assay optimization phase. The results from the assay optimization phase will determine whether further validation steps are required. These additional requirements vary according to the needs of your specimens.